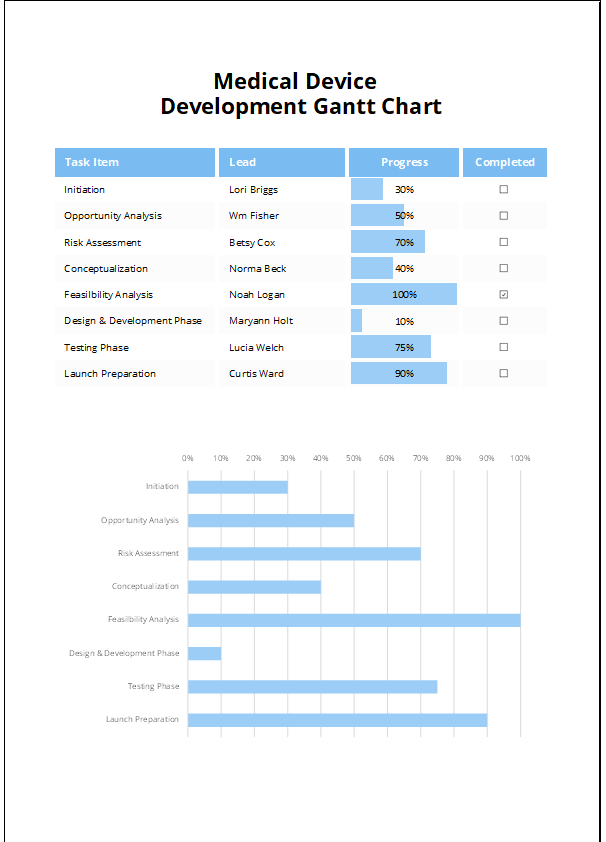
The Medical Device Development Gantt Chart Template is a professional Excel tool built to help teams plan, track, and manage medical device development timelines. It supports every stage of development—from concept to regulatory approval—with a structured visual timeline. Whether you’re launching a new device or updating an existing one, this Gantt chart keeps your team organized and compliant with industry standards.
This template is ideal for R&D managers, regulatory teams, quality assurance leads, and product development teams in medtech companies.
Key Features and Functionalities
The template includes a dynamic Gantt chart that automatically updates when you input task start and end dates. Each stage appears as a color-coded bar, showing what’s pending, active, or complete. A built-in progress tracker helps teams monitor timelines and meet critical deadlines.
Users can customize task names, durations, responsible departments, and development phases. The layout works for both early-stage research and final product launch timelines. It also supports coordination across cross-functional teams and external partners.
Use Cases of this Gantt Chart Template
The Medical Device Development Gantt Chart is suitable for a variety of high-stakes workflows, including:
- Concept development: Track research, feasibility studies, and early risk assessments.
- Prototyping: Manage design iterations, component testing, and functional validation.
- Preclinical studies: Schedule laboratory testing and animal study timelines.
- Regulatory planning: Organize submissions to FDA, CE Marking, or other global authorities.
- Clinical trials: Track patient recruitment, data collection, and trial reporting.
- Design verification and validation: Plan testing, documentation, and quality reviews.
- Manufacturing scale-up: Coordinate production readiness, supplier management, and packaging design.
This tool is built to support compliance with ISO 13485, FDA guidelines, and other industry frameworks.
Download this Gantt Chart Template
The Medical Device Development Gantt Chart Template helps streamline complex development processes. It keeps teams aligned, improves transparency, and ensures all regulatory and technical milestones are met on time. With this Excel chart, your team can manage timelines with confidence and control.
Download now and guide your medical device project from idea to launch using this structured Gantt chart template.